Tigan (trimethobenzamide hydrochloride), injectable
Tigan® (trimethobenzamide hydrochloride), injectable
Product Summary
Therapeutic Class: Antiemetics This product is manufactured and distributed by MCP Pharmaceuticals, LLC
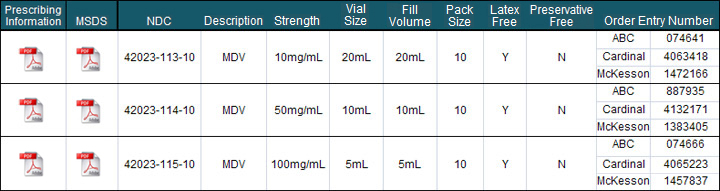
Product Profile:
- Latex Status:
- Tigan 20mL vial stopper contains latex
- Tigan 2mL vial stopper is latex free
- Preservative Status:
- Tigan 20mL MDV contains 0.45% phenol as a preservative
- Tigan 2mL SDV is preservative free
- Bar Coded
Description
Each Tigan® 2-mL single-dose vial contains 200 mg trimethobenzamide hydrochloride compounded with 1 mg sodium citrate and 0.4 mg citric acid as buffers and pH adjusted to approximately 5.0 with sodium hydroxide. For Tigan® multi-dose vials, each mL contains 100 mg trimethobenzamide hydrochloride compounded with 0.45% phenol as preservative, 0.5 mg sodium citrate and 0.2 mg citric acid as buffers and pH adjusted to approximately 5.0 with sodium hydroxide.
Indications and Usage
Tigan® is indicated for the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis.
Contraindications
The injectable form of Tigan® is contraindicated in pediatric patients and in patients with known hypersensitivity to trimethobenzamide.
Warnings
Tigan® may produce drowsiness. Patients should not operate motor vehicles or other dangerous machinery until their individual responses have been determined.
Usage In Pregnancy
Trimethobenzamide hydrochloride was studied in reproduction experiments in rats and rabbits and no teratogenicity was suggested. The only effects observed were an increased percentage of embryonic resorptions or stillborn pups in rats administered 20 mg and 100 mg/kg and increased resorptions in rabbits receiving 100 mg/kg. In each study these adverse effects were attributed to one or two dams. The relevance to humans is not known. Since there is no adequate experience in pregnant or lactating women who have received this drug, safety in pregnancy or in nursing mothers has not been established.
Usage With Alcohol
Concomitant use of alcohol with Tigan® may result in an adverse drug interaction.
Adverse Events
There have been reports of hypersensitivity reactions and Parkinson-like symptoms. There have been instances of hypotension reported following parenteral administration to surgical patients. There have been reports of blood dyscrasias, blurring of vision, coma, convulsions, depression of mood, diarrhea, disorientation, dizziness, drowsiness, headache, jaundice, muscle cramps and opisthotonos. If these occur, the administration of the drug should be discontinued. Allergic-type skin reactions have been observed; therefore, the drug should be discontinued at the first sign of sensitization. While these symptoms will usually disappear spontaneously, symptomatic treatment may be indicated in some cases.
Please see the full prescribing information link above for additional information.
| MK117C |