Dantrium Intravenous (IV) (dantrolene sodium for injection)
Dantrium® Intravenous (IV) (dantrolene sodium for injection)
Product Summary
Therapeutic Class: Skeletal Muscle Relaxant This product is manufactured and distributed by MCP Pharmaceuticals, LLC
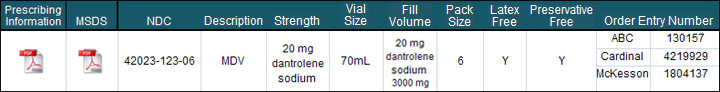
Product Profile:
- Latex Free
- Preservative Free
- Bar Coded
For additional detailed information on product and resources, please visit the Dantrium® IV Product Website at www.dantrium.com Malignant hyperthermia (MH), an event triggered in susceptible individuals by various anesthetics, is a sudden and life threatening condition. The Malignant Hyperthermia Association of the United States (MHAUS) recommends a minimum stocking level of 36 vials of dantrolene sodium for injection (Dantrium® IV) for facilities that use triggering agents. This number is based on the amount of Dantrium® IV used to treat an MH episode for the average adult (70 kg). However, the size of the patient and the severity of the reaction will dictate the number of vials needed. If you would like to order educational materials on how to prepare your facility for an MH crisis, please contact MHAUS at 800-98-MHAUS.
Description
Dantrium® IV is a sterile, non-pyrogenic, lyophilized formulation of dantrolene sodium for injection. Dantrium® IV is supplied in 70 mL vials containing 20 mg dantrolene sodium, 3000 mg mannitol, and sufficient sodium hydroxide to yield a pH of approximately 9.5 when reconstituted with 60 mL sterile water for injection USP (without a bacteriostatic agent).
Indications and Usage
Dantrium® IV is indicated, along with appropriate supportive measures, for the management of the fulminant hypermetabolism of skeletal muscle characteristic of malignant hyperthermia crisis in patients of all ages.
Dantrium® IV is also indicated preoperatively, and sometimes postoperatively, to prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia in individuals judged to be malignant hyperthermia susceptible.
Contraindications
None.
Warnings
The use of Dantrium® IV in the management of malignant hyperthermia crisis is not a substitute for previously known supportive measures. These measures must be individualized, but it will usually be necessary to discontinue the suspect triggering agents, attend to increased oxygen requirements, manage the metabolic acidosis, institute cooling when necessary, monitor urinary output, and monitor for electrolyte imbalance.
Adverse Events
There have been occasional reports of death following malignant hyperthermia crisis even when treated with intravenous dantrolene. Adverse events with Dantrium® IV include loss of grip strength, weakness in the legs, drowsiness, dizziness, thrombophlebitis, and tissue necrosis/injection site reactions secondary to extravasation. There have been rare reports of pulmonary edema, uticaria and erythema.
Symptomatic hepatitis (fatal and non-fatal) has been reported at various dose levels of the drug. Fatal and non-fatal liver disorders of an idiosyncratic or hypersensitivity type may occur with Dantrium® therapy. In case of overdose, symptoms include, but are not limited to, muscular weakness, lethargy, coma, vomiting, diarrhea, and crystalluria. For acute overdosage, general supportive measures should be employed.
1 Malignant Hyperthermia Association of the United States Website. Retrieved March 23 2011.
Please see the full prescribing information link above for additional information.
| MK130C |