MCP Pharmaceuticals Launches New Multipack Presentations of Adrenalin®, Coly-Mycin® M and Pitocin®
PARSIPPANY, NJ July 05, 2011 — MCP Pharmaceuticals, a leading marketer and manufacturer of sterile injectable products, is launching larger pack sizes for three products commonly used in acute care clinical settings: Adrenalin® (epinephrine injection, USP), Coly-Mycin® M Parenteral (colistimethate sodium, USP) and Pitocin® (oxytocin injection, USP synthetic).
Stuart Hinchen, CEO and President of MCP noted, “The larger pack size was developed to meet customer demand for cost savings and increased pharmacy efficiency by reducing packaging waste. Our new multipacks allow pharmacy staff to open one carton containing multiple vials rather than open multiple cartons containing only one vial. The efficiency is critical in busy pharmacies handling high volume products such as Adrenalin®, Coly-Mycin® M and Pitocin® and the cost savings are significant.”
Commenting on MCP’s philosophy of marketing mature brand name products at competitive prices, Hinchen stated, “MCP is focused on meeting three key customer needs: quality, price and supply. Reducing packaging waste in high volume products is an example of how we stay price competitive without sacrificing quality. We are also vigilant in our efforts to provide the market with a reliable drug supply. Since we manufacture our products, we have the dexterity to adapt to changing market demand which is crucial in today’s injectable market.”
All three products are still available in single vial packs to meet the needs of smaller clinical settings. For more information about these products or other MCP products, please visit jhppharma.com/jhppricing and contact your local wholesaler or distributor.
| Products Available in a New Multipack Size* |
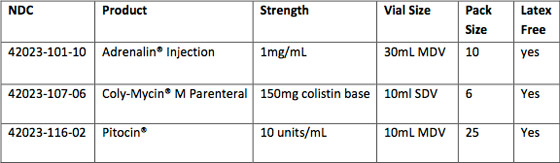 |
About MCP Pharmaceuticals, LLC
MCP Pharmaceuticals, headquartered in New Jersey, is an integrated specialty healthcare company that acquires, develops, manufactures and sells sterile injectable products. MCP markets 15 well-established branded pharmaceutical products primarily into the hospital segment. MCP provides customers with a reliable supply of brand name products at competitive prices.
MCP employs more than 330 staff in the USA in its manufacturing, product development, sales & marketing and corporate areas. For more information, please visit jhppharma.com.
Additional Press Releases
 |
MCP Pharmaceuticals Enters Agreement To Produce Life-Saving Drug Webpage | PDF Format |
 |
MCP Pharmaceuticals Announces Chief Executive Officer Succession Webpage | PDF Format |
 |
MCP Pharmaceuticals Launches New Multipack Presentations of Adrenalin®, Coly-Mycin® M and Pitocin® Webpage | PDF Format |
 |
MCP Pharmaceuticals Announces Multi-Year Contract Manufacturing Agreement Webpage | PDF Format |
 |
MCP Pharmaceuticals Enters Another Multi-Year Contract Manufacturing Agreement Webpage | PDF Format |
 |
MCP Pharmaceuticals & Putney Inc. Announce Multi-Year Contract Manufacturing Agreement Webpage | PDF Format |
 |
MCP Pharmaceuticals Announces Multi-Year Contract Manufacturing Agreement Webpage | PDF Format |
 |
MCP Pharmaceuticals Announces Successful Completion of FDA and EMEA GMP Audits Webpage | PDF Format |
 |
MCP Announces FDA Approval for Dantrium IV, rapidly mixing in 20 seconds Webpage | PDF Format |
 |
MCP Pharmaceuticals & SpePharm complete purchase of Dantrium® (dantrolene sodium) Webpage | PDF Format |
 |
King Pharmaceuticals Completes Sale Of Rochester, Michigan Manufacturing Facility To MCP Pharmaceuticals Webpage | PDF Format |
Return To Press & News »
